THE ENT database
- Clinical documentation
- Quality assurance
- Research
Now with ZCMEI
- www.entstatistics.com
Advertisement
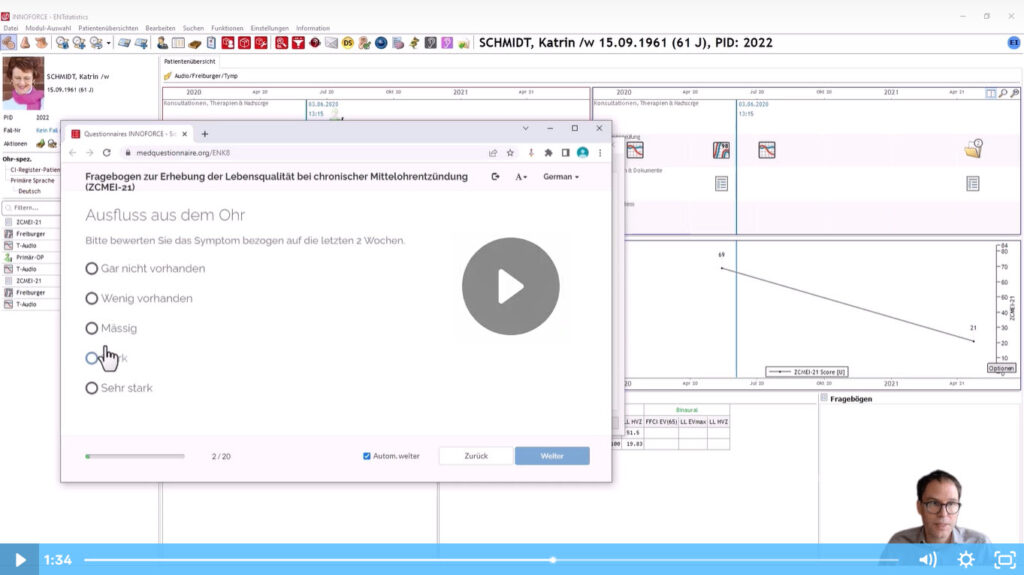
In this video you can see how the ZCMEI questionnaire is integrated into ENTstatistics and how patients can easily complete the questionnaire on a tablet or smartphone. The Video is narrated in GERMAN.
Measuring Health-Related Quality of Life in Chronic Otitis Media
Get the Zurich Chronic Middle Ear Inventory (ZCMEI-21) and its validated translations. free to use
Interested in conducting a translation and validation study of the ZCMEI-21 in a new language not yet available?

THE ENT database
- Clinical documentation
- Quality assurance
- Research
Now with SAMEO-ATO
- www.entstatistics.com
Advertisement
In this video you can see how the ZCMEI questionnaire is integrated into ENTstatistics and how patients can easily complete the questionnaire on a tablet or smartphone. The Video is narrated in GERMAN.
About
The ZCMEI-21 is a globally acknowledged disease-specific questionnaire employed to assess health-related quality of life in chronic otitis media. It consists of four subscores that evaluate ear-related symptoms, hearing ability, psychosocial impacts of the disease, and utilization of medical resources. Responses are recorded on a 5-point Likert scale and scored from 0 to 4. The maximum total score for the ZCMEI-21 is 84, with higher scores indicating a lower health-related quality of life.
The ZCMEI-21 has been translated and validated in multiple languages, such as English, Italian, French, Japanese, Chinese, Turkish and others. The availability of this disease-specific patient-reported outcome measure in various languages facilitates the standardization of health-related quality of life assessments on an international scale.
Notably, the ZCMEI-21 stands out as the sole globally recognized disease-specific patient-reported outcome measure for chronic otitis media with an established “Minimal Clinically Important Difference” (MCID). The MCID serves as a crucial threshold, indicating a value beyond which a change in the total score of a questionnaire – post-therapy for instance – holds subjective significance for the patient. For the ZCMEI-21, the MCID is set at 5 points, meaning that a statistically significant alteration in the ZCMEI-21 total score carries clinical significance if it reaches or surpasses 5 points.
Selected References
Development, Validation, and Translations of the ZCMEI-21
- Maesschalck T, Quatre R, Horoi M, Rodriguez A, Sagnet P, Röösli C, Huber A, Voruz F, Senn P, Schmerber S, Bächinger D. A novel instrument for assessing health-related quality of life in French patients: translation and multi-centre validation of the Zurich chronic middle ear inventory (ZCMEI-21-Fr). Eur Arch Otorhinolaryngol. 2025;282(10):5123-5129. doi:10.1007/s00405-025-09447-0
- Bächinger D, Röösli C, Ditzen B, Huber AM. Development and validation of the Zurich chronic middle ear inventory (ZCMEI-21): an electronic questionnaire for assessing quality of life in patients with chronic otitis media. Eur Arch Otorhinolaryngol. 2016 Oct;273(10):3073-81. doi: 10.1007/s00405-016-3915-7.
- Chatzimichalis M, Epprecht L, Weder S, Shaul C, Engle Folchert KJ, Machala MC, Goosmann MM, Naville M, Zhu A, Röösli C, Lee DJ, Cass SP, Briggs R, Huber AM, Bächinger D. English translation and validation of the Zurich chronic middle ear inventory (ZCMEI-21-E) assessing quality of life in chronic otitis media: A prospective international multicentre study. Clin Otolaryngol. 2019 May;44(3):254-262. doi: 10.1111/coa.13275.
- Ralli M, Quaranta N, Canale A, Röösli C, Milella C, De Robertis V, De Soccio G, Greco A, Ralli G, Albera R, de Vincentiis M, Huber AM, Bächinger D. Cross- cultural Adaption and Validation of the Zurich Chronic Middle Ear Inventory Translated Into Italian (ZCMEI-21-It)-a Prospective Multicenter Study. Otol Neurotol. 2019 Mar;40(3):351-358. doi: 10.1097/MAO.0000000000002131.
- Bächinger D, Takagi D, Yamada H, Teraoka M, Okada M, Hyodo J, Röösli C, Huber AM, Hato N. Japanese translation, cross-cultural adaption and multicentre validation of the Zurich chronic middle ear inventory (ZCMEI-21-Jap). Auris Nasus Larynx. 2019 Feb;46(1):18-23. doi: 10.1016/j.anl.2018.05.008. Epub 2018 Jun 2. PMID: 29871811.
- Yang R, Zhang Y, Han W, Li Y, Li S, Ke J, Song Y, Liu J, Röösli C, Huber AM, Bächinger D, Ma F. Measuring health-related quality of life in chronic otitis media in a Chinese population: cultural adaption and validation of the Zurich Chronic Middle Ear Inventory (ZCMEI-21-Chn). Health Qual Life Outcomes. 2020 Jul 8;18(1):218. doi: 10.1186/s12955-020-01461-6.
- Tutar B, Saltürk Z, Berkiten G, Ekincioğlu ME, Karaketir S, Arkan ME, Akgün MF, Yılmazer AB, Kulak E, Baechinger D, Uyar Y. A novel Turkish instrument for assessing quality of life in chronic otitis media –translation and validation of Zurich chronic middle ear inventory. Turk J Med Sci. 2020 Dec 17;50(8):1922-1929. doi: 10.3906/sag-2003-38.
Minimal Clinically Important Difference of the ZCMEI-21
- Yang R, Zhang Y, Feng G, Han W, Li Y, Li S, Pan T, Ke J, Zhang K, Xin Y, Song Y, Zuo Q, Zhao Y, Zhou N, Yao Z, Röösli C, Huber AM, Bächinger D, Ma F, Gao Z. Determining the Minimal Clinically Important Difference (MCID) and Responsiveness of the Chinese Version of Zurich Chronic Middle Ear Inventory (ZCMEI-21-Chn): A Prospective Multicenter Study. Otol Neurotol. 2024;45(7):e532-e540. doi:10.1097/MAO.0000000000004237
- Bächinger D, Mlynski R, Weiss NM. Establishing the minimal clinically important difference (MCID) of the Zurich Chronic Middle Ear Inventory (ZCMEI-21) in patients treated for chronic middle ear disease. Eur Arch Otorhinolaryngol. 2020 Apr;277(4):1039-1044. doi: 10.1007/s00405-020-05819-w. Epub 2020 Jan 27. PMID: 31989271; PMCID: PMC7072039.
Applications of the ZCMEI-21
- Raemy Y, Bächinger D, Peter N, Roosli C. Health-related quality of life in patients after endoscopic or microscopic cholesteatoma surgery. Eur Arch Otorhinolaryngol. 2024. doi:10.1007/s00405-024-09097-8.
- Müller C, Raczynski A, Lailach S, Zahnert T. Conventional one-handed compared to two-handed endoscopic ear surgery using an endoscope holder: a single center study. Eur Arch Otorhinolaryngol. 2024 Oct. doi: 10.1007/s00405-024-09018-9.
- Weiss N, Stallbaum T, Botzen J, Bächinger D, Großmann W, Bernd HE, Mlynski R. Allogenes und autologes Material führt bei Mastoidhöhlenobliteration zu vergleichbaren Rezidivraten [Mastoid cavity obliteration with allogenic and autologous material]. Laryngorhinootologie. 2022 Jan;101(1):40-44. German. doi: 10.1055/a-1432-3123.
- Mlynski R, Bächinger D, Langanke T, Lailach S, Neudert M, Weiss NM. Comparison of two disease‑specific instruments assessing health-related quality of life in patients with chronic otitis media. Eur Arch Otorhinolaryngol. 2022 Feb;279(2):703-711. doi: 10.1007/s00405-021-06702-y.
- Bächinger D, Großmann W, Mlynski R, Weiss NM. Characteristics of health-related quality of life in different types of chronic middle ear disease. Eur Arch Otorhinolaryngol. 2021 Oct;278(10):3795-3800. doi: 10.1007/s00405-020-06487-6.
- Lailach S, Langanke T, Zahnert T, Garthus-Niegel S, Neudert M. Impact of depressive disorders on quality of life after middle ear surgery in patients with chronic otitis media. Eur Arch Otorhinolaryngol. 2021 Sep;278(9):3217-3225. doi: 10.1007/s00405-020-06397-7.
- Weiss NM, Bächinger D, Rrahmani A, Bernd HE, Huber A, Mlynski R, Röösli C. Mapping the ChOLE classification to hearing outcomes and disease-specific health-related quality of life. Eur Arch Otorhinolaryngol. 2020 Oct;277(10):2729-2738. doi: 10.1007/s00405-020-06002-x.
- Weiss NM, Bächinger D, Botzen J, Großmann W, Mlynski R. Mastoid cavity obliteration leads to a clinically significant improvement in health-related quality of life. Eur Arch Otorhinolaryngol. 2020 Jun;277(6):1637-1643. doi: 10.1007/s00405-020-05881-4.
- Weiss NM, Stecher S, Bächinger D, Schuldt T, Langner S, Zonnur S, Mlynski R, Schraven SP. Open Mastoid Cavity Obliteration With a High-Porosity Hydroxyapatite Ceramic Leads to High Rate of Revision Surgery and Insufficient Cavity Obliteration. Otol Neurotol. 2020;41(1):e55–e63. doi:10.1097/MAO.0000000000002413.
Reviews on Measuring Health-related Quality of Life in Chronic Otitis Media
- Devi NS, Kumar A, G V, Sood R, Tyagi AK, Jat B, Patro S, Jayaprakash PA, Prasath R, Yadav AC, Nivedhan R. Assessment of Quality-of-Life Measurement Instruments for Chronic Otitis Media: A Systematic Review Using the COnsensus-Based Standards for the Selection of Health Measurement Instruments (COSMIN) Checklist. Indian J Otolaryngol Head Neck Surg. 2025;77(2):699-710. doi:10.1007/s12070-024-05224-3
- Devi NS, Kumar A, Vetrivel G, Sood R, Tyagi AK, Jat B, Patro S, Jayaprakash PA, Prasath R, Yadav AC, Nivedhan R. Assessment of quality-of-life measurement instruments for chronic otitis media: a systematic review using the COnsensus-based Standards for the Selection of Health Measurement Instruments (COSMIN) checklist. Indian J Otolaryngol Head Neck Surg. 2024. doi:10.1007/s12070-024-05224-3.
- Bächinger D, Neudert M, Dazert S, Röösli C, Huber A, Mlynski R, Weiss NM. Gesundheitsbezogene Lebensqualität bei der chronischen Otitis media – Messmethoden und deren Anwendung bei chirurgischen Therapien [Health-related quality of life in chronic otitis media-measurement methods and their application in surgical therapy]. HNO. 2023 Sep;71(9):556-565. German. doi: 10.1007/s00106-023-01324-8.
- Schouwenaar EMM, Hellingman CA, Waterval JJ. Health-related quality of life after otologic surgical treatment for chronic otitis media: systematic review. Front Neurol. 2023;14:1268785. doi: 10.3389/fneur.2023.1268785.
- Lucidi D, Cantaffa C, Nocini R, Martone A, Alicandri-Ciufelli M, Marchioni D, Presutti L, Molinari G. Quality of Life after Surgical Treatment for Chronic Otitis Media: A Systematic Review of the Literature. J Pers Med. 2022 Nov 27;12(12):1959. doi: 10.3390/jpm12121959.
- Lailach S, Baumann I, Zahnert T, Neudert M. Aktueller Stand der Lebensqualitätsmessung bei Patienten mit chronischer Otitis media und Schallleitungsschwerhörigkeit [State of the art of quality-of-life measurement in patients with chronic otitis media and conductive hearing loss]. HNO. 2018 Aug;66(8):578-589. German. doi: 10.1007/s00106-018-0524-3.
Contact
If you have any questions or comments, we are happy to hear from you!
Website development and maintenance
by INNOFORCE Est